

Sterilization, while highly effective, is not usually reversible all other methods are reversible, most immediately upon stopping them. The least effective methods are spermicides and withdrawal by the male before ejaculation. Less effective methods include physical barriers such as condoms, diaphragms and birth control sponges and fertility awareness methods. This is followed by a number of hormone-based methods including oral pills, patches, vaginal rings, and injections. The most effective methods of birth control are sterilization by means of vasectomy in males and tubal ligation in females, intrauterine devices (IUDs), and implantable birth control. The World Health Organization and United States Centers for Disease Control and Prevention provide guidance on the safety of birth control methods among women with specific medical conditions. The term birth control is a bit of a misnomer since abortion is not regularly considered under the term. Some cultures limit or discourage access to birth control because they consider it to be morally, religiously, or politically undesirable. Planning, making available, and using birth control is called family planning. Birth control has been used since ancient times, but effective and safe methods of birth control only became available in the 20th century. Transvaginal ultrasound (TVU) should not be used as the Essure Confirmation Test, as TVU cannot confirm tubal occlusion.Birth control, also known as contraception, anticonception, and fertility control, is a method or device used to prevent pregnancy. For these patients, physicians must use the modified HSG as the Essure Confirmation Test. Patients on immunosuppressive therapy may experience delay or failure of the necessary tissue in-growth for tubal occlusion.Currently there is no test that reliably predicts who may develop a hypersensitivity reaction to the materials contained in the insert. Patients should be counseled on the materials contained in the insert prior to the Essure procedure. Symptoms reported for this device that may be associated with an allergic reaction include hives, urticaria, rash, angioedema, facial edema and pruritus. In addition, some patients may develop an allergy to nickel or other components of the insert following placement.
Patients with known hypersensitivity to nickel, titanium, platinum, stainless steel, and PET (polyethylene terephthalate) fiber or any of the components of the Essure system may experience an allergic reaction to the insert.This is also more likely to occur in individuals with a history of pain. Pain (acute or persistent) of varying intensity and length of time may occur following Essure placement.The Essure procedure should be considered irreversible.The sale and distribution of this device are restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Bayer.
Iud birth control pdf info manual#
Device to be used only by physicians who are knowledgeable hysteroscopists have read and understood the Instructions for Use and Physician Training Manual and have successfully completed the Essure training program, including preceptoring in placement until competency is established, typically 5 cases.
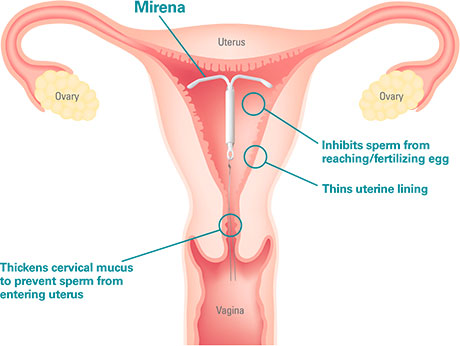
WARNING: Some patients implanted with the Essure System for Permanent Birth Control have experienced and/or reported adverse events, including perforation of the uterus and/or fallopian tubes, identification of inserts in the abdominal or pelvic cavity, persistent pain, and suspected allergic or hypersensitivity reactions.


 0 kommentar(er)
0 kommentar(er)
